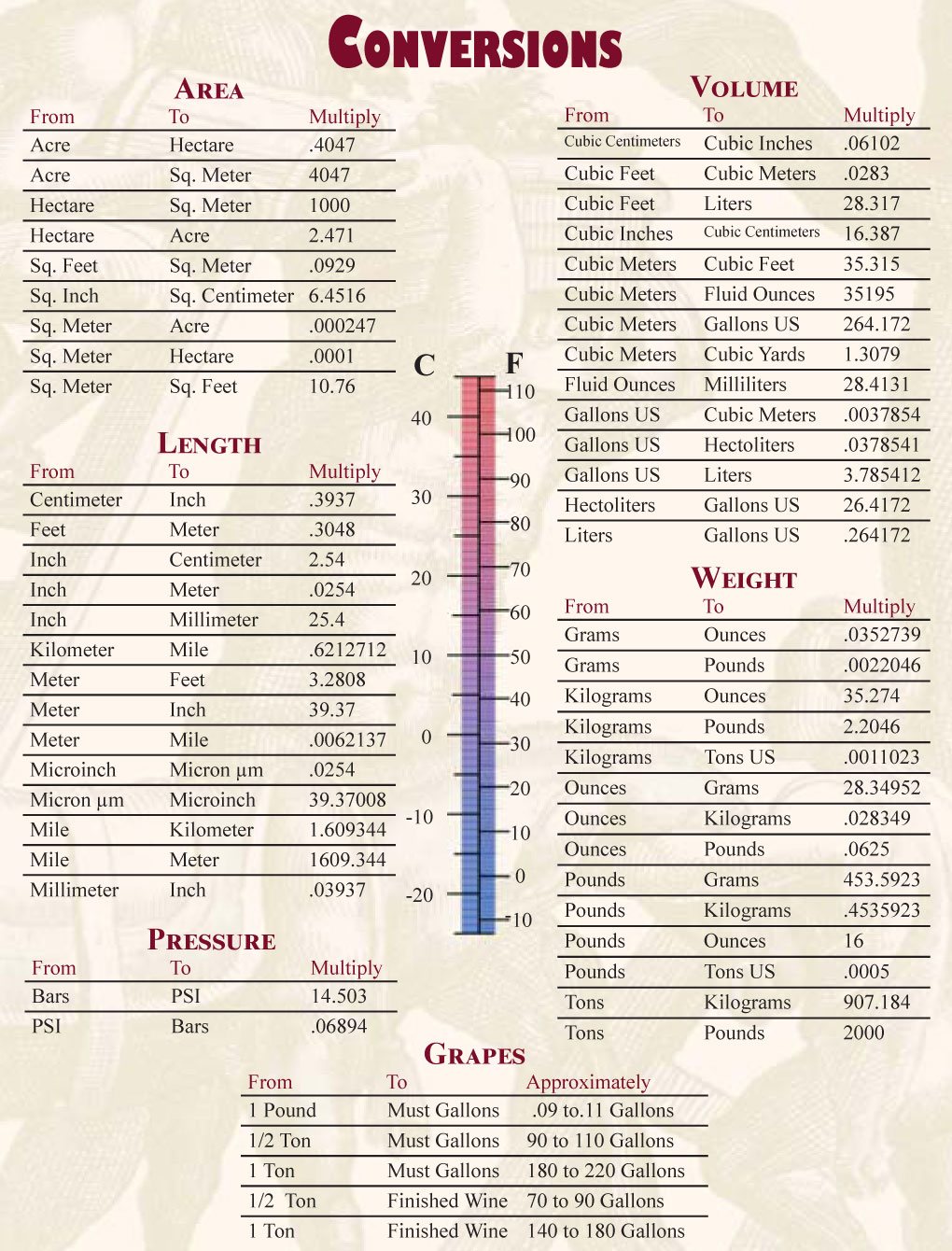
Wine Making Formulas & Conversions
Warning: The Below Formulas are for Reference Only, Actual Results May Vary
(Especially if you ask other Winemakers)
| From | To | Approximately |
| 1 pound | Must Gallons | 0.9 to .11 Gallons |
| 1/2 Ton | Must Gallons | 90 to 110 Gallons |
| 1 Ton | Must Gallons | 180 to 220 Gallons |
| 1/2 Ton | Finished Wine | 70 to 90 Gallons |
| 1 Ton | Finished Wine | 140 to 180 Gallons |
Volume of Wine in a Case
To calculate the number of Cases, you will need the total gallons or total liters
Standard Case (750 mL) = 9 Liters or 2.376 Gallons
Case of Splits (375 mL) = 4.5 Liters or 1.188 Gallons
Standard Barrel (59 gal) = 25 Cases
Ordering Labels
Most North American Bottling Lines and Labeling Machines do Front and Back Labels from a Single Spool. Because Labelers will eat some labels, it is advised when ordering labels to use:
Under 10,000 Cases = 14 Labels per Case
Over 10,000 Cases = 13.5 Labels per Case
Common Label Winding Chart
.jpg)
Acid Additions and Reductions
These formulas are general guidelines and are not recommended for real application.
Following the Basic Titration Formula will always provide more accurate results!
To Increase TA by 0.1% add: To Decrease TA by 0.1% add:
1.0 grams tartaric acid per liter of wine 2.5 grams per gallon calcium carbonate
3.8 grams tartaric acid per gallon of wine 3.8 grams per gallon potassium carbonate
Basic Titration Formula
Make a 10% solution of an addition. Take a 100 mL sample of your wine
Titrate using 10% solution drop by drop until desired pH is reached
Taste sample and if you like the results follow the formula X = Y
X = grams per liter Y = mL of 10% solution used to reach desired pH
Bentonite Additions For Fining X = Y x 0.4
X = Grams of Bentonite to be added per liter of wine
Y = Liters of Wine to Treat
Cleaning Formulas
Citric Acid = 70 grams per gallon
TSP = 70 grams per gallon
Proxi-Clean = 28.4 grams per gallon
Iodine = 17 mL per gallon
Caustic Soda = 36 grams per gallon
Copper Sulfate Additions X = Y x 0.2
X = mL of 1% Copper Sulfate Solution to produce about 0.1 ppm of Copper in the wine
Y = Gallons of Wine to treat
Making a 10 % Solution
Weigh out 10 grams of product and place product in a 100 mL Volumetric Flask
Fill Volumetric Flask to 100mL with Distilled Water, Gently Shake Till Completely Dissolved
Potential Alcohol Range Formula
Brix x 0.55 = Low Alcohol Potential
Brix x 0.59 = High Alcohol Potential
PPM of Sulfur Dioxide
To calculate parts per million (ppm) of Sulfur Dioxide with Potassium Metabisulfite:
1 gram = 150 ppm in 1 gallon, 30 ppm in 5 gallons
1/4 teaspoon (1.5 grams) = 225 ppm in 1 gallon or 45 ppm in 5 gallons
Molecular Stability in Relation to pH
This following chart shows the desired molecular stability levels in red and white wines. In red wines keeping a .5 molecular stability helps to avoid issues and keeps red wines stable. However if you should notice an issue within the red wines such as increasing VA levels, bring the wine to a .8 molecular stability.
This being said unless absolutely necessary you should avoid SO2 levels above 60 ppm free for ageing. Levels higher than 60 ppm will effect the wines taste and aromatics. Also it is best to avoid levels above 40 ppm when bottling or for regular consumption of the wine.
| Free SO2 Needed to Achieve | ||
| Whites | Reds | |
| pH | .8 Molecular | .5 Molecular |
| 2.9 | 11 ppm | 7 ppm |
| 3.0 | 13 ppm | 8 ppm |
| 3.1 | 16 ppm | 10 ppm |
| 3.2 | 21 ppm | 13 ppm |
| 3.3 | 26 ppm | 16 ppm |
| 3.4 | 32 ppm | 20 ppm |
| 3.5 | 40 ppm | 25 ppm |
| 3.6 | 50 ppm | 31 ppm |
| 3.7 | 63 ppm | 39 ppm |
SO2 Addition Formula X = (Y - A) x 3 / 2 / 1000 x 3.785 x Z/ 60
To find the appropriate addition, take your desired SO2 Level (Y) and subtract your current SO2 Level (A) in your wine. Multiply this number by 3 then divide by 2. This is to compensate for the 1/3 of the addition which will bind instantly when you make the addition. From here divide this number by 1000 then multiply it by 3.785 (Liters in a gallon); multiply this number by the volume of your wine in gallons (Z) then divide this by 60. This final number is your addition of a 10% solution of Potassium Metabisulfite in liters. If you number come out 0.135 it is equal to 0.135 liters or 135 ml. (Equivalent to 13.5 grams) (Note this formula is not for sterile filtered wines)
X = Liters of a 10% Solution of Potassium Metabisulfite
Y = Desired ppm - Current ppm
A = Current SO2 ppm
Z = Amount of Wine in Gallons
Comparison of Fining Agents
(Regarding desired effects & potential problems listed in order of most effective to least effective)
| Color Reduction | Tannin Reduction | Clarity & Stability |
| Carbon | Gelatin | Bentonite |
| Gelatin | Albumen | Alginic Acid |
| Casein | Isinglass | Carbon |
| PVPP | PVPP | Casein |
| Isinglass | Bentonite | Gelatin |
| Alginic Acid | Alginic Acid | Albumen |
| Potential for Overfining | Quality Impairment | Volume of Lees Formed |
| Gelatin | Carbon | Bentonite |
| Carbon | Gelatin | Gelatin |
| Albumen | Bentonite | Casein |
| Isinglass | Casein | Albumen |
| Casein | Albumen | Isinglass |
| PVPP | Isinglass | Alginic Acid |
| Alginic Acid | PVPP | PVPP |
| Bentonite | Alginic Acid | Carbon |
Egg Albumin
Dissolve Egg Albumin Powder in 10 times its weight in water (1 Kg / 10 liters of water) Never dissolve the preparation directly in the wine: this will provoke immediate flocculation with the tannins in the wine. Mix vigorously using a whisk, avoid foam formation and lumps. It is essential to mix the product rapidly with the wine to obtain good dispersion.
5 to 10 g/hL (4 g/hl of Egg Albumin Powder corresponds to 1 fresh egg white)
Bench Trials
There are many types of fining agents available but all products should be bench trialed prior to making the addition to your wine. Most packages will instruct you in additional ranges from 1 to 6 lbs per 1000 gallons. A quick reference for bench trials:
| Additions per Gallon | Addition per Liter |
| 0.5 g = 1.1 lbs per 1000 Gallons | 0.12 g = 1 lbs per 1000 Gallons |
| 1.0 g = 2.2 lbs per 1000 Gallons | 0.24 g = 2 lbs per 1000 Gallons |
| 1.5 g = 3.3 lbs per 1000 Gallons | 0.36 g = 3 lbs per 1000 Gallons |
| 2.0 g = 4.4 lbs per 1000 Gallons | 0.48 g = 4 lbs per 1000 Gallons |
| 2.5 g = 5.5 lbs per 1000 Gallons | 0.60 g = 5 lbs per 1000 Gallons |
| 3.0 g = 6.6 lbs per 1000 Gallons | 0.72 g = 6 lbs per 1000 Gallons |
Sulfur Dioxide (SO2) Reduction with Hydrogen Peroxide (H2O2)
To reduce Sulfur Dioxide by 10 parts per million:
Add 1.0 mL of 3% Hydrogen Peroxide per gallon of must or wine
Titratable Acid Formula
g/l = (ml NaOH x Normality NaOH) x 75
_______________________________
Sample Volume (mL)
Vacuum Aspiration Formula
Free SO2 (ppm) = N NaOH x mls NaOH x 1600
Example: If N of NaOH is 0.01, then: Free SO2 (ppm) = mls NaOH x 16
Volume of Alcoholic Spirit Required Formula X = V x ( C-A) / B - C
X = the volume of fortifying spirit required in liters
V = the volume of must or wine to be fortified in liters
C = the final alcohol content aimed for
A = the alcohol content of the fermenting wine at fortification
B = the alcohol strength of the fortifying spirit
Volume of Wine in a Vertical Tank
If you need to determine how many gallons are in a partially full tank use the following formula
You will need to measure Diameter of the Tank and the Height of Fluid in the Tank
For Measuring in Feet use: X = 3.14 x Y / 4 x H x 7.48
For Measuring in Inches use: X = 3.14 x Y / 4 x H x 0.004328
X = Gallons of Wine in the Tank
Y = Diameter of the Tank Squared ex: 6’ Diameter Tank = 36’ Diameter Squared (6’ x 6’ = 36’)
H = Height of Wine in the Tank 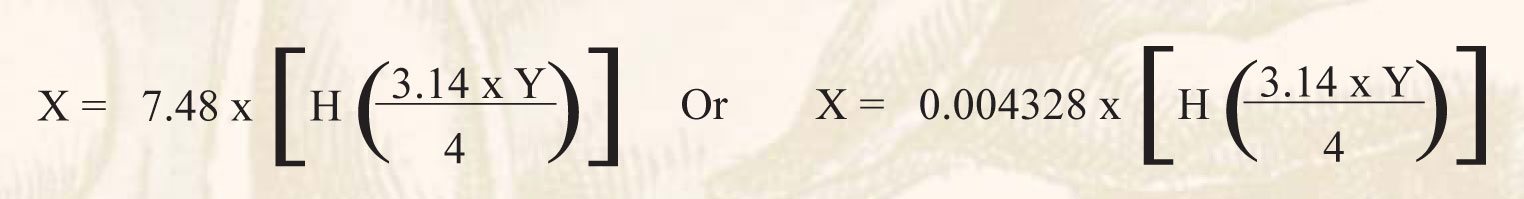
Adding Water to High Sugar Grapes L1 x O / B = L2 then L2 - L1 = Y
You must first determine how much finished wine you will produce before you dilute your must or juice. For white grape juice, your yield is roughly the same as your starting volume. In general, for red grape musts, the yield is 3 1/2 gallons finished, pressed wine per 5 U.S. gallons of fermented must.
O = original brix of must or juice
L1 = volume (in liters) of finished wine from undiluted must/juice
B = brix you want to dilute must/juice to
L2 = volume (in liters) of finished wine from diluted must/juice
Y = volume (in liters) of water to add to must or juice to dilute to desired level, B.
Tapered Stopper Sizes |
||||||
| Stopper # | Length | Top Diameter | Bottom Diameter | |||
| Inches | mm | Inches | mm | Inches | mm | |
| 00 | 1/2 | 12.7 | 5/16 | 7.9 | 7/32 | 5.5 |
| 0 | 1/2 | 12.7 | 3/8 | 9.5 | 9/32 | 7.1 |
| 1 | 5/8 | 15.9 | 7/16 | 11.1 | 21/64 | 8.3 |
| 2 | 11/16 | 17.5 | 1/2 | 12.7 | 3/8 | 9.5 |
| 3 | 3/4 | 19.0 | 9/16 | 14.3 | 27/64 | 10.7 |
| 4 | 13/16 | 20.6 | 5/8 | 15.9 | 15/32 | 11.9 |
| 5 | 7/8 | 22.2 | 11/16 | 17.5 | 17/32 | 13.5 |
| 6 | 15/16 | 23.8 | 3/4 | 19.0 | 37/64 | 14.7 |
| 7 | 1 | 25.4 | 13/16 | 20.6 | 5/8 | 15.9 |
| 8 | 1-1/16 | 27.0 | 7/8 | 22.2 | 43/64 | 17.1 |
| 9 | 1-1/8 | 28.6 | 15/16 | 23.8 | 47/64 | 18.6 |
| 10 | 1-1/4 | 31.8 | 1 | 25.4 | 49/64 | 19.4 |
This page is currently under Construction...
 Winery
Winery Distillery
Distillery Brewery
Brewery Olive Oil
Olive Oil